Home > Video archives
Video archives
Here, we establish an online video library where a series of movies relevant to motility are available. The miscellaneous category includes bacteria, eukaryotes, and archaea, viruses, proteins, and synthetic polymers. The movies that are meaningful in the biology field will be uploaded in both Japanese and English.
For the contributors who plan to upload your video, you should keep in mind the following suggestions:
(1) the video which is relative to the object of your research
(2) the video about microbe found in the research activity of the super-science high school or biological clubs are encouraged to upload
(3) Do not forget to add the link of your video which has been published (Please make sure the copyright)
(4) If you think some videos in the old textbook are valuable to upload, please let us know.
Video List
 1
1
Eukaryote number of click:427
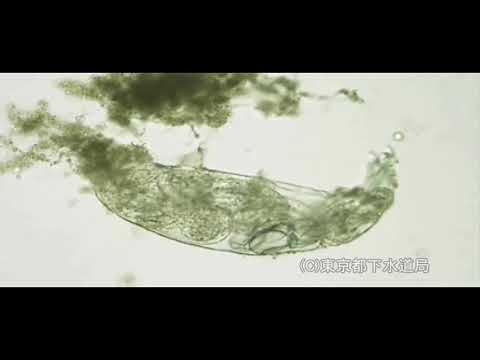
Philodina sp.
Species name:Philodina sp.
Bureau Swerage, Tokyo Metropolitan Government
The size of Philodina is 300-1,000 μm in length. The body is thin long. One foot with four toes. The head has two crown of chilia. The eyespots lie on the brain. It moves like leeches. It shows leech-like movement and moves around flocs. Philodina feed on algae and bacteria. It can retract cron of cilia into the body.
 2
2
Eukaryote number of click:371
Pollen tube attraction by the synergid cell
Species name:Torenia fournieri
ITbM, Nagoya Univ Tetsuya Higashiyama
In the evolution of flowering plants, genes necessary for flagella formation including flagellar dyneins were lost. Non-motile sperm cells of flowering plants are conveyed by a tip-growing haploid cell, the pollen tube. The sperm cell is enclosed by an endocytic membrane of the pollen tube cell and delivered to female gametes rapidly without much water for swimming. How does the pollen tube precisely arrive at an egg-containing tissue? Pollen tube attractants had been searched for more than 140 years. The attractants were finally identified in a unique plant species, Torenia, which has a protruding egg-containing tissue. Pollen tube attraction can be directly observed in Torenia as shown in this movie. Two synergid cells on the side of the egg cell were shown to be the source of the attraction signal. Finally, two cysteine-rich peptides named LUREs were identified as true pollen tube attractants.
 3
3
Eukaryote number of click:225
Mouse tracheal cilia
Species name:Mus musculus
Hamamatsu Univ Sch Med Koji Ikegami
Moving cilia on mouse tracheal epithelia. The beating frequency is about 10 to 20 Hz. The batch of cilia shows a wavelike motility, so-called metachronal wave.
4
Prokaryote number of click:217
Bacterium moves like a tank 3
Species name:Flavobacterium johnsoniae
Gakushuin University Daisuke Nakane
Flavobacterium johnsoniae and many other members of Bacteroidetes exhibit rapid gliding motility over surfaces by a unique mechanism. These cells do not have flagella or pili; instead, they rely on a novel motility apparatus. SprB, a 669 kDa cell-surface adhesin, is required for efficient gliding. Here, we showed dynamic movements of SprB were observed by fluorescent microscopy. SprB moved at a constant speed of 2 um/s on the cell surface along a left-handed helical closed loop, appears that the cell have a moving conveyer belt. Attachment of SprB to the substratum was associated with cell movement, suggesting a model for gliding, in which adhesins are propelled along a helical track, generating rotation and translation of the cell.
5
Eukaryote number of click:204
Mysterious behavior of Bacillaria
Species name:Bacillaria paxillifer
AL-Museum AL-Museum
Bacillaria is a colony in which numerous individual diatoms are connected. The individual diatoms are lined up side by side, which looks like a window blind when the colony is contracted. When the colony stretches out, the diatoms are connected nearly end to end in a long chain-like structure. Bacillaria usually moves in a straight line when extended, but can change directions freely when contracted. Various small diatoms, aggregates and crystals are stuck to Bacillaria and move along with it.
6
Eukaryote number of click:197
Macrobiotus sp.
Species name:Macrobiotus sp.
Tokyo Metropolitan Government Bureau Swerage,
The size of Macrobiotus is about 0.2 – 1.0 mm. The body is covered with a thin chitin film, with spiny bristles, armor plates. There are four pairs of footsteps, with nails at the tip. Macrobiotus have fourth pair of legs with nails at the tip. They usually live in soil. The form that I'm slow in action and walk slowly is similar to a bear, so it's called a bear bug. The tooth needle taken out of the mouth is stuck into food, and it's crowded, and a pharynx, to work, more, I suck at contents. Slowly walking figure resembles a bear so it is called water bears.
7
Eukaryote number of click:196
Cell cycle-uncoupled cytokinesis in AmiA:myosin II double KO cells.
Species name:Dictyostelium discoideum
National Institute of Advanced Industrial Science and Technology Taro Uyeda
The mutant cellular slime mold Dictyostelium discoideum lacking myosin II and AmiA cannot perform cell cycle-dependent cytokinesis. These cells fragment by traction-mediated, cell cycle-uncoupled method of division (cytokinesis C). These cells express GFP-histone to visualize nuclear division. Note that nuclear division, synchronous in each multinucleate cell, is not followed by cell division.
8
Prokaryote number of click:195
Swimming Rhodobacter spheroids (1)
Species name:Rhodobacter spheroids
Harvard University Howard C. Berg
Department of Molecular and Cellular Biology
Harvard Biological Labs
Swimming Rhodobacter sphaeroides
9
Molecule and Protein number of click:187
Movement of Actin-associated Myosin-II (Cross-Bridge) during Muscle Contraction
Species name:Rabbit
Osaka City Univ Eisaku KATAYAMA
The first part of the movie indicates the movement of individual myosin-head (crossbridge), based on conventional "Tilting-Leverarm Hypothesis". Such movement was proposed from the characteristic features of the atomic models of myosin-S1 in the absence and the presence of ATP, together with the well-known experimental evidence that "the motor-domain does not appreciably rotate during the Power-Stroke". Hence, this hypothesis claims that the Power-Stroke is essentially the transition between strongly actin-bound rigor-structure (1DFK: lever-arm is extended) and ATP-bound kinked structure (1DFL). If the motor-domain is immobilized on actin, the lever-arm moiety should swing along the actin filament, The latter half of the movie exhibits the revised crossbridge-cycle we have proposed according to our direct observation of in vitro sliding actomyosin by Quick-Freeze Deep-Etch Replica Electron Microscopy (Ref. 1, 2). We noticed that the actual images of actin-sliding myosin cannot be explained by the conventional hypothesis as above, suggesting the presence of a new conformer whose crystal structure is not yet reported. After extensive search, we finally found that SH1-SH2 crosslinked myosin could be a good candidate of the new conformer whose lever-arm bends to the opposite side of ATP-bound kinked structure (Ref.3-5). Since we could successfully reconstruct its low-resolution 3-D model by a new version of single-particle-analysis (Ref. 5). Taking the results of time-resolved chemical crosslinking into consideration, we revised the scheme of crossbridge-cycle including the new conformer (Ref.5). .The conformational change shown in the movie is compatible with all the images we actually observed under in vitro actin-sliding conditions. [References] 1. Katayama E. The effects of various nucleotides on the structure of actin-attached myosin subfragment-1 studied by quick-freeze deep-etch electron microscopy. J Biochem. 1989 Nov;106(5):751-70. 2: Katayama E. Quick-freeze deep-etch electron microscopy of the actin-heavy meromyosin complex during the in vitro motility assay. J Mol Biol. 1998 May 1;278(2):349-67. 3: Katayama E, Ohmori G, Baba N. Three-dimensional image analysis of myosin head in function as captured by quick-freeze deep-etch replica electron microscopy. Adv Exp Med Biol. 1998;453:37-45. 4: Katayama E, Ichise N, Yaeguchi N, Yoshizawa T, Maruta S, Baba N. Three-dimensional structural analysis of individual myosin heads under various functional states. Adv Exp Med Biol. 2003;538:295-304. 5: Kimori Y, Baba N, Katayama E. Novel configuration of a myosin II transient intermediate analogue revealed by quick-freeze deep-etch replica electron microscopy. Biochem J. 2013 Feb 15;450(1):23-35. 6. Andreev OA, Reshetnyak YK. Mechanism of formation of actomyosin interface. J Mol Biol. 2007 Jan 19;365(3):551-4.
10
Eukaryote number of click:187
Trypanosoma brucei bloodstream form
Species name:Trypanosoma brucei
Institute of Cell Biology, University of Bern, Switzerland Prof. Torsten Ochsenreiter
Trypanosoma brucei (Mitat1.1) is a single celled protozoan parasite that causes Human african trypanomiasis and Nagana in cattle. The movie is in slow motion the cells actually swim much faster. Images were captured by Dr. Torsten Ochsenreiter using a Zeiss Cell Observer Microscope (63x DIC objective) at the University of Georgia, Athens, USA.